New research from Johns Hopkins Medicine suggests an experimental cancer drug could enhance standard tuberculosis (TB) treatment and help prevent long-term lung damage. The study, “Proapoptotic Bcl-2 Inhibitor As Potential Host-Directed Therapy for Pulmonary Tuberculosis,” was published in Nature Communications.
The drug, navitoclax, is a Bcl-2 inhibitor that is currently in clinical trials to treat cancer by accelerating programmed cell death. Although researchers said it has no individual effect on TB, it increases effectiveness when combined with standard TB antibiotics rifampin, isoniazid and pyrazinamide (RHZ).
This discovery could transform TB therapy as more people become resistant to customary antibiotics. Researchers also believe it could help prevent post-TB lung dysfunction, a known risk for millions of TB patients.
“Current treatment regimens for TB are lengthy, expensive and leave patients vulnerable to relapse and lung scarring. Our research shows that adding in a host-directed therapy has extraordinary promise to solve these problems,” said senior author Sanjay Jain, MD, in a news release. Dr. Jain is a pediatric infectious disease specialist at Johns Hopkins Children’s Center and professor of pediatrics in the Johns Hopkins University School of Medicine.
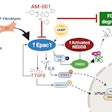
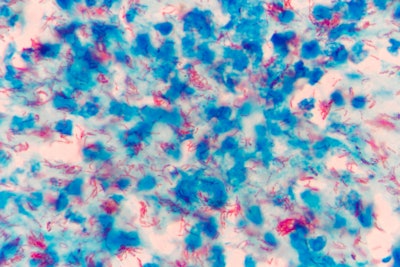
According to the study’s first author Medha Singh, PhD, a pediatric infectious diseases fellow at the university, Mycobacterium tuberculosis hijacks otherwise healthy molecular pathways and prompts infected host cells to produce Bcl-2 — a protein that plays a critical role in regulating cell death (apoptosis). This activity triggers a state of pulmonary necrosis in which bacteria multiple and the immune system fails to attack and kill the cells. Navitoclax helps prevent this destructive process.
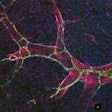
The study was first of its kind to test the Bcl-2 inhibitor as an add-on treatment for TB. The research group utilized clinically translatable positron emission tomography (PET) technology to collect and analyze images of the mouse models.
During the four-week course of treatment, infected mice that received RHZ and navitoclax, compared to those that only received RHZ, experienced the following:
- A 40% reduction in necrotic pulmonary lesions
- A 40% reduction in lung scarring
- Double the amount of pulmonary apoptosis
- A decreased risk of the infection spreading to other organs, such as the spleen
- A decreased bacterial burden
Researchers said the results suggest navitoclax could produce similar effects in patients with other chronic bacterial infections, such as non-TB mycobacteria and Staphylococcus aureus.
If additional trials are successful, Dr. Jain said navitoclax or a similar drug could be added to the standard TB antibiotic regiment. This would shorten the typical daily six-month treatment process, reduce the prevalence of lung scarring or post-TB lung disease and improve outcomes for patients with drug-resistant TB.