Researchers from Weill Cornell Medicine in New York City have confirmed the role PAR1 plays in healing lung injuries caused by infection, trauma or toxins. The protein PAR1 helps lymphatic vessels structurally transform to boost fluid drainage and clear inflammation. Previously, the underlying mechanism of this process was unknown, the authors reported in their study, “The Thrombin Receptor PAR1 Orchestrates Changes in Lymphatic Endothelial Cell Junction Morphology to Augment Lymphatic Drainage during Lung Injury.”
Published in Nature Cardiovascular Research, the study demonstrated that PAR1 triggers a change in the spaces between endothelial cells, which line the inside of lymphatic vessels of the lungs. This transformation makes the vessels permeable, so they can absorb more fluid and immune cells — a response that is distinct from blood vessels, where similar changes result in leakage and disease — the authors noted.
 Hasina Outtz Reed, MD, PhDWeill Cornell Medicine
Hasina Outtz Reed, MD, PhDWeill Cornell Medicine
Until recently, lung lymphatics have been understudied, said Dr. Outtz Reed, who is assistant professor of pulmonary and critical care medicine at Weill Cornell Medicine and a pulmonologist at New York-Presbyterian/Weill Cornell Medical Center.
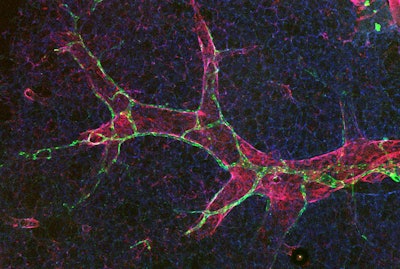
The research team examined mouse models and observed how the junctions between endothelial cells — referred to as buttons and zippers — change in the lungs’ lymphatic vessels. The button junctions are permeable, allowing lymphatic vessels to take up fluid and cells. In contrast, the zipper junctions are tight and don’t allow fluid to enter the vessels.
“One of the more surprising findings was that a large percentage of the lung lymphatic endothelial cells were zipped. But in response to injury, zippered junctions in the lungs can rapidly reorganize to be buttoned, aiding in fluid uptake,” Dr. Outtz Reed said.
Findings from the study suggest that without PAR1, the lymphatic vessels remain stuck in zipper mode, even when the lungs are inflamed. This results in decreased fluid drainage, a buildup of immune cells and worsening inflammation. The researchers found that a biochemical signal triggered the zipper-to-button switch.
Therefore, therapies that block PAR1, like some cancer or cardiovascular drugs, have implications in inflammatory lung diseases.
“A lot of clinical trials targeting PAR1 have been unsuccessful. We think, in part, this may be due to the lymphatic vasculature, which also expresses this receptor but responds to the drug in completely different ways than intended,” Dr. Outtz Reed said.
The researchers plan to explore how the changes in lymphatic junctions impact the lungs’ response to infectious agents, such as viruses and bacteria. Dr. Outtz Reed said she will also investigate how to target PAR1 on the lung vessels while sparing the blood vessels.