In a recent experiment of mice infected with COVID-19, investigators identified rare lung immune cells that prove essential to regulating inflammation caused by the deadly virus. The new study, “Nerve- and Airway-Associated Interstitial Macrophages Mitigate SARS-CoV-2 Pathogenesis Via Interferon Signaling,” was published in the journal Immunity.
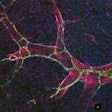
Researchers from NYU Langone Health led the experiment that analyzed the unique immune cell type called nerve- and airway-associated interstitial macrophages (NAMs). Macrophages are the first cells to respond to infection and are capable of consuming viruses and infected cells.
According to the study’s authors, the breakthrough shifts the focus for treating the disease to limiting the body’s immune response (known as disease tolerance) rather than strengthening the immune system to attack the virus.
The research group tested the impact of NAMs in two groups of mice infected with the SARS-CoV-2 virus. Mice with deficient NAMs experienced more viral spread, increased inflammation, weight loss and eventually death. Mice with intact NAMs experienced fewer effects and all survived.
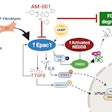
While most immune cell sets taper off over time, the researchers found that NAMs propagate. NAMs also were shown to restrict the production of highly pro-inflammatory signaling proteins to prevent tissue damage.
“Our findings underscore the critical role of nerve- and airway-associated interstitial macrophages in the lungs in regulating the inflammatory response during SARS-CoV-2 infection,” said Payal Damani-Yokota, PhD, co-lead investigator and postdoctoral fellow at NYU Grossman School of Medicine, in a press release. “The absence of these NAM immune cells leads to an exaggerated inflammatory response against the COVID-19 virus, cell death and increased viral spread.”
The results also showed that NAMs rely on a protein called type 1 interferon receptor (IFNAR) to have this immune response effect on SARS-CoV-2. When investigators genetically engineered NAMs to stop producing IFNAR, the cells could no longer control inflammation, and the mice died.
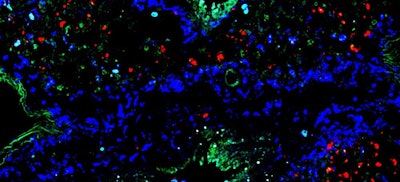
To confirm the outcomes of the mice experiment, researchers analyzed lung tissue from patients who had been intubated due to severe COVID-19 infection. Individuals who died had significantly less activity in NAM-related genes as well as heightened inflammation compared to individuals who survived.
The same NYU Langone team initially classified NAMs in 2020, which led to this recent study to validate its theory of the immune cell type’s role in controlling inflammation in COVID-19.
“The new work unveils nerve- and airway-associated interstitial macrophages as key players in the choreography of recovery — poised to silence the alarms of inflammation and restore calm, even in the midst of interferon’s call to amplify the immune response,” said Benjamin tenOever, PhD, the Jan T. Vilcek Professor of Molecular Pathogenesis and chair of the department of microbiology at NYU Langone.
The investigators said they plan to further examine NAM pathways to determine how the macrophage subset moderates inflammation and investigate type 1 interferon signaling to understand how it triggers NAM growth in response to SARS-CoV-2 infection.
Senior study investigator Kamal M. Khanna, PhD, said the research could help develop treatment strategies that harness IFNAR signaling to promote disease tolerance — an approach that could apply to COVID-19 as well as other respiratory conditions, including COPD, asthma and pulmonary fibrosis. Dr. Khanna is an associate professor in the department of microbiology at NYU Grossman School of Medicine.