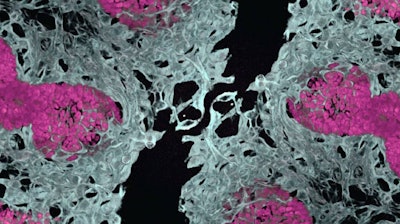
In a major leap forward for regenerative medicine, researchers at Cincinnati Children’s Center for Stem Cell & Organoid Medicine (CuSTOM) in Ohio have successfully developed human lung and gut organoids that can grow their own blood vessels — an achievement that has been years in the making and could revolutionize how scientists study disease and potentially treat organ failure. CuSTOM is a multidisciplinary research center focused on using stem cells and organoids to advance personalized medicine and model human diseases.
The study, “Co-Development of Mesoderm and Endoderm Enables Organotypic Vascularization in Lung and Gut Organoids,” was recently published in the journal, Cell. It marks the first time that organotypic vasculature (blood vessels that mimic those found in real human organs) has been integrated into lung organoids derived from induced pluripotent stem cells (iPSCs).
“Prior to our study, the development of lung organoids with organotypic vasculature had not been achieved,” said co-corresponding author Mingxia Gu, MD, PhD, in a press release. Dr. Gu is now vice chair of research at the University of California Los Angeles David Geffen School of Medicine. “Notably, this method also could be applied to other organ systems such as intestine and colon.”
Creating organoids involves converting mature human cells (such as blood or tissue cells) back into fetal-like stem cells that can be coaxed into growing a wide range of other tissue types. Unlike disconnected human cells kept alive in a dish, these are growing, developing mini-organs that form into seed-sized spheres that mimic the unique functions of full-sized organs.
Researchers used a novel approach to co-differentiate mesoderm and endoderm cells within the same spheroid, guided by bone morphogenetic protein (BMP) signaling. This allowed them to fine-tune the balance of endothelial and epithelial progenitors, essential for forming realistic lung and intestinal tissue, Dr. Gu said.
Using single-cell RNA sequencing, the team identified organ-specific gene signatures and key ligands that drive endothelial development. The vascularized organoids not only matured more effectively but also integrated with host circulation when transplanted into mice, preserving their lung or gut identity.
“The challenge in vascularizing endodermal organs, particularly the lung, stems from different signaling requirements for lung epithelial versus vascular differentiation,” said study co-author Yifei Miao, PhD, who is now at the Beijing Institute for Stem Cell and Regenerative Medicine in China. “Our success in this endeavor is attributable to our unique differentiation method.”
According to Dr. Gu, the research addresses a long-standing challenge in organoid science: the inability to form internal blood vessels. Without vasculature, organoids remain limited in size and function, she said. These vascularized models can:
- Mimic human fetal lung and intestine development
- Support gas exchange and alveolar formation
- Model diseases like alveolar capillary dysplasia with misalignment of pulmonary veins (ACDMPV), a fatal condition in newborns
- Offer insights into endothelial-epithelial crosstalk, especially in disorders involving FOXF1 mutations
CuSTOM has filed patents for its methods and is continuing to refine the technology. Its work opens the door to custom-grown tissues that could one day repair damaged organs or replace animal models in drug testing.
“We look forward to applying these discoveries to improve outcomes across a wide range of difficult human diseases,” said co-director of CuSTOM Aaron Zorn, PhD.