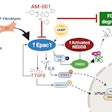
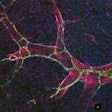
Researchers at Boston Medical Center and Boston University have developed an innovative method for generating human alveolar epithelial type I cells (AT1s) from pluripotent stem cells (iPSCs). The results of the novel study, published in Cell Stem Cell, provide an in vitro model of human AT1 cells.
AT1s, which line much of the gas exchange barrier of the distal lung, are a potential source to developing regenerative therapies but have been historically difficult to isolate. Now, using the iPSC-based model, researchers can recreate and analyze these cells in greater detail and be able to advance their understanding of human lung regeneration, specifically after an infection or exposure to toxins.
This approach could also help expedite progress in treatment and therapeutic options for people with pulmonary conditions or disorders of the alveolar epithelium, such as acute respiratory distress syndrome (ARDS) and pulmonary fibrosis.
“Uncovering the ability to generate human alveolar epithelial type I cells (AT1s), and similar cell types, from pluripotent stem cells (iPSCs) has expanded our knowledge of biological processes and can significantly improve disease understanding and management,” said Darrell Kotton, MD, director, Center for Regenerative Medicine (CReM) of Boston University and Boston Medical Center.
According to Dr. Kotton, the study’s results also further the CReM's goal of generating every human lung cell type from iPSCs as a pathway to improving disease management and provide a foundation of cells for future transplantation to regenerate damaged lung tissues in vivo.
“We know that the respiratory system can respond to injury and regenerate lost or damaged cells, but the depth of that knowledge is currently limited,” said first author Claire Burgess, PhD, a post-doctoral fellow at Boston University Chobanian and Avedisian School of Medicine. “We anticipate this protocol will be used to further understand how AT1 cells react to toxins, bacteria and viral exposures, and will be used in basic developmental studies, disease modeling and potential engineering of future regenerative therapies.”