Ocean Biomedical, Inc. plans to target OCF-203, its pulmonary fibrosis treatment candidate as a novel therapeutic for fatal pulmonary fibrotic conditions caused by Hermansky-Pudlak Syndrome (HPS). Jack A. Elias, MD, Ocean Biomedical’s co-founder, and colleagues at Brown University, have revealed a new target and a new pathway for treating pulmonary tissue damage in idiopathic pulmonary fibrosis (IPF). Experiments have also been performed with genetically modified “pale-ear” mice, which have the same mutations that are seen in patients with HPS. These experiments demonstrated that the same small molecule may be effective in treating pulmonary fibrosis conditions in patients with HPS, especially the most deadly forms of that disease, HPS-1 and HPS-4.
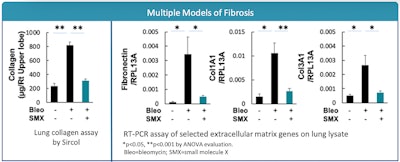
Ocean Biomedical’s approach to treating pulmonary fibrosis, a condition with no disease-modifying agents currently available, is focused on inhibiting Chitinase 1 (Chit1) with patented OCF-203. Chit1 is also a critical biomarker in scleroderma-associated interstitial lung disease (SSc-ILD) and plays a role in bleomycin- and IL-13-induced pulmonary fibrosis. In four pulmonary fibrosis animal models, Ocean Biomedical’s OCF-203 has shown an 85-90% reduction in collagen accumulation. The results of this antifibrotic (termed molecule X: SMX) in the bleomycin model can be seen in the diagram above. Results also showed efficacy in the pale-ear mouse model of HPS, including reductions in fibrosis.
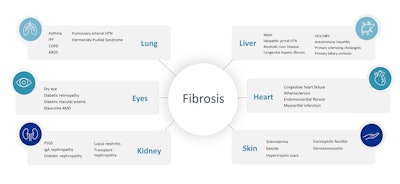
Ocean Biomedical’s anti-fibrosis platform seeks to address major unmet needs for IPF and HPS and has shown potential for expanded application into fibrotic diseases in other organs, such as scleroderma, alcoholic liver disease, NASH and kidney failure.
HPS is a rare genetic condition that affects about one in 750,000 people worldwide, and about one in 1,800 people in northwest Puerto Rico. In HPS-1 and HPS-4 patients, pulmonary fibrosis occurs early in life (30s and 40s) and symptoms are often severe. Patients who have the HPS-1 or HPS-4 variants of the disease often develop terminal lung fibrosis with no therapeutic treatment currently approved.
“My life’s work has been focused on caring for patients with pulmonary conditions, and it is difficult to see the limited treatment options that are available for many of these diseases. We are working to change that, and we’re really excited about the broad therapeutic potential for this unique treatment pathway,” said Dr. Elias.