Clinical stage biotechnology company aTyr has completed enrollment for its phase 3 EFZO-FIT trial. The global study will assess the safety and efficacy of efzofitimod for treating adult patients with pulmonary sarcoidosis, a major form of interstitial lung disease (ILD).
The company exceeded its enrollment goals, accepting 268 patients at 85 centers in nine countries. It expects to release the topline results in mid- to late-2025.
“Completing enrollment in this landmark study is an important milestone that brings us one step closer to delivering a potentially groundbreaking treatment to address the unmet need for pulmonary sarcoidosis patients,” said Sanjay S. Shukla, MD, MS, president and CEO of aTyr, in a press release.
EFZO-FIT is a 52-week, multicenter, randomized, double-blind, placebo-controlled study. Participants will receive intravenous efzofitimod (either 3 mg/kg or 5 mg/kg) or placebo for a total of 12 doses. The use of forced steroid will taper throughout the study.
“It is by far the largest interventional study ever to be conducted in sarcoidosis. We expect the results of this trial to yield valuable insights that will inform sarcoidosis research and treatment in the years to come,” said lead investigator Daniel A. Culver, DO, chair of the Division of Pulmonary Medicine at The Cleveland Clinic. “We are optimistic, based on the positive phase 1b/2a results, that efzofitimod could be a potentially transformative therapy.”
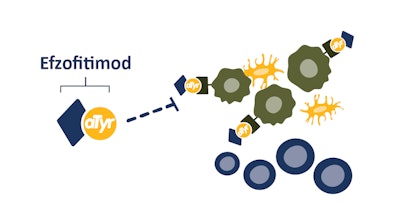
Current treatment options for pulmonary sarcoidosis are limited and invasive. Long-term use of anti-inflammatory and immunosuppressive corticosteroids can cause severe side effects. Efzofitimod is a first-in-class biologic immunomodulator that selectively modulates activated myeloid cells through neuropilin-2 receptor protein (NRP2). The tRNA synthetase-derived therapy was developed to reduce inflammation without suppressing the immune system and prevent the progression of fibrosis.
The primary endpoint of the phase 3 EFZO-FIT trial is to lower oral corticosteroid dosage in patients with the condition. Secondary endpoints include improvement in lung function and symptoms compared to placebo.
Efzofitimod has orphan drug designation in the United States, European Union and Japan, as well as Fast Track designation from the U.S. Food and Drug Administration (FDA) for pulmonary sarcoidosis.