Researchers at UC Davis Health completed a multicenter safety study testing a new protein drug (INBRX-101). The experimental drug received U.S Food and Drug Administration (FDA) Fast Track designation in May 2023 for the treatment of patients with emphysema due to alpha-1 antitrypsin deficiency (AATD).
Results from the phase 1 trial were published in the Journal of the COPD Foundation and showed the drug therapy to be dependable, with minimal side effects.
 Brooks Kuhn, MD, MASUC Davis Health
Brooks Kuhn, MD, MASUC Davis Health
AATD is a rare genetic disease caused by insufficient levels of the AAT (alpha-1) protein. Patients who have AATD experience loss of lung tissue and function that can be fatal.
“Alpha-1 is supposed to come in and tell neutrophils the war is over,” said Dr. Kuhn in a UC Davis Health interview. “But when a patient is deficient, they have unchecked, persistent neutrophilic inflammation. Because the lungs are at the front lines of exposure, they typically take the hardest hits.”
Currently, the standard treatment for AATD is weekly infusions of plasma-derived AAT, which can be invasive and time-consuming. Patients who relied on this therapy struggled during the COVID-19 pandemic when there was a shortage of plasma donations.
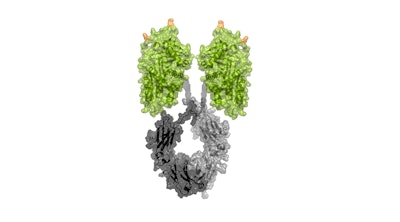
INBRX-101 combines alpha-1 proteins with an antibody that allows the drug to remain longer in a person’s blood and organs. Because of this increased protection, the early results suggest that it could be given intravenously every three to four weeks. Also, the drug’s synthetic formation should avoid issues in supply chain.
“I was impressed by how clean the results were,” Dr. Kuhn said. “The pharmacokinetics, or how long a drug is effective in the body, turned out great and the immunogenicity was minimal. There’s real promise there and, for the AATD patients I treat with infusion, I don’t think I can oversell the practical benefits for them.”
The research team will continue to review initial findings and use the data to prepare for a phase 2/3 trial.