A study unveiled by Olympus has shown sustained clinical benefits of its Spiration Valve System (SVS) in treating patients suffering with severe symptoms of emphysema, a form of COPD.
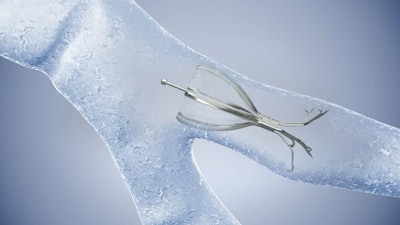
The Spiration Valve is an umbrella-shaped device that is placed in the most diseased parts of the lung during a short bronchoscopic procedure. Treatment with endobronchial valves such as the Spiration Valve can improve lung function by redirecting air away from hyperinflated portions of the lung to healthier portions.
The EMPROVE study is the first randomized controlled trial to present a rigorous comparison of bronchoscopic lung volume reduction using endobronchial valve with standard-of-care controls for up to 24 months in severe emphysema patients.
Researchers found no difference in adverse events between the SVS treatment and control groups, and there were no reported cases of migration or erosion in the SVS treatment group at 24 months.
“This study points to the significant and positive long-term impact SVS treatment can have on the day-to-day functions of emphysema patients,” said John de Csepel, chief medical officer at Olympus Corporation. “Meaningful improvement in breathing can mean fuller lives for patients for activities as simple as the ability to go on daily walks or enjoying time with grandchildren.”
EMPROVE, a prospective, multicenter, randomized controlled trial, included 172 patients from 31 sites in the United States and Canada. Patients with severe heterogeneous emphysema were randomly assigned treatment with the Spiration Valve System or to the control group.