German biotechnology company BioNTech announced the official launch of its first-in-human mRNA lung cancer vaccine trial. The LuCa-MERIT-1 study will evaluate the safety and efficacy of the interventional immunotherapy (known as BNT116) to treat and prevent recurrence of non-small cell lung cancer (NSCLC).
The innovative vaccine uses mRNA (messenger RNA), the same technology employed in the COVID-19 vaccines, to activate a person’s immune system to identify lung tumor markers and attack the cancerous cells. Unlike chemotherapy, which kills both harmful and healthy cells, BTN116 will only target cancer cells with the expressed biomarkers.
The trial aims to establish the safety profile of BNT116, including tolerable doses for monotherapy and combination therapy with cemiplimab or docetaxel. So far, the trial has enrolled 130 patients representing various stages of NSCLC, from early to late stages of the disease with or without treatment of initial diagnosis or recurrent cancer.
Study participants come from 34 research sites across seven countries: Germany, Hungary, Poland, Spain, Turkey, the United Kingdom and the United States.
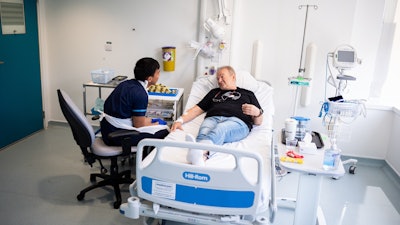
Janusz Racz, 67 years old, was the first patient to receive the novel cancer vaccine in late August 2024. He is receiving his care at University College London Hospitals (UCLH).
“I thought it over, and … decided to take part because I hope it will provide a defense against cancer cells. But I also thought that my participation in this research could help other people in the future and help this therapy become more widely available,” said Racz in a UCLH news release.
According to ClinicalTrials.gov, there are approximately 70 trials worldwide currently enrolling or preparing to recruit participants for mRNA-based cancer vaccines.