The U.S. Food and Drug Administration (FDA) has granted breakthrough device designation for a tuberculosis (TB) screening tool that is powered by artificial intelligence (AI). Medical imaging company Qure.ai received the clearance on Feb. 5, 2024, for its qSpot-TB solution. It also received the European Union Medical Device Regulation (EU MDR) CE mark.
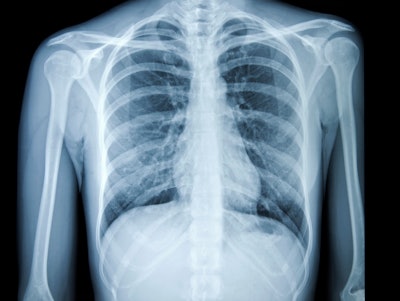
QSpot-TB is an innovative second-read computer that aids in the detection and diagnosis of tuberculosis. Using AI, the tool analyzes chest X-rays to identify all noted radiological signs indicative of TB and confirm its presence.
In the United States, the incidence of TB had been consistently declining for the past three decades. However, its presence has resurged since 2021. The Centers for Disease Control and Prevention (CDC) reported more than 8,300 tuberculosis cases in 2022.
"The increase in TB cases in the U.S. is a reminder about the importance of collective global efforts to continue the fight against the disease until eliminated,” said Professor Kenneth G. Castro, MD, a co-director of the Emory Tuberculosis Center and professor of global health, epidemiology and infectious disease at the Rollins School of Public Health at Emory University in Atlanta. “We cannot let our guard down. Innovative technology is a crucial component for accelerated progress to successfully end TB globally.”
The designation for qSpot-TB makes for a total of four FDA designations and sixty-one EU MDR CE mark approvals for Qure.ai in the last eighteen months.
“Qure is committed to pushing the boundaries of AI medical innovation and follows rigorous legislative and regulatory processes in over eighty-five countries to ensure the highest standards of safety and efficacy of solutions,” said Bunty Kundnani, chief regulatory affairs officer at Qure.ai. “Achieving FDA and EU MDR clearances across multiple imaging modalities and global disease areas means that we can confidently support radiology workforces to prioritize patient cases quickly or expedite decision making.”