A researcher at Boston University has received a $3.1 million grant for a project aimed at using artificial intelligence (AI) to overcome limitations of current diagnostic tools when it comes to COPD.
The project, “Integrating Multi-Modal Data and Biomechanics in COPD: Toward Robust and Interpretable Biomarkers for Disease Subtyping and Progression,” will be led by Kayhan Batmanghelich, PhD, assistant professor of electrical and computer engineering and Hariri Institute Junior Faculty Fellow at Boston University.
The project will also include researchers from the University of Pittsburgh School of Medicine and Brigham and Women’s Hospital.
“Lung function tests and CT scans are standard for diagnosing COPD, but they don’t capture how the disease progresses,” Dr. Batmanghelich said in a news release. “Current radiomics lack sensitivity, and most deep learning models are limited, hard to interpret and sensitive to scan variations.”
The research team will address these challenges by building on their previous work, “DrasCLR: A Self-Supervised Framework of Learning Disease-Related and Anatomy-Specific Representation for 3D Lung CT Images.”
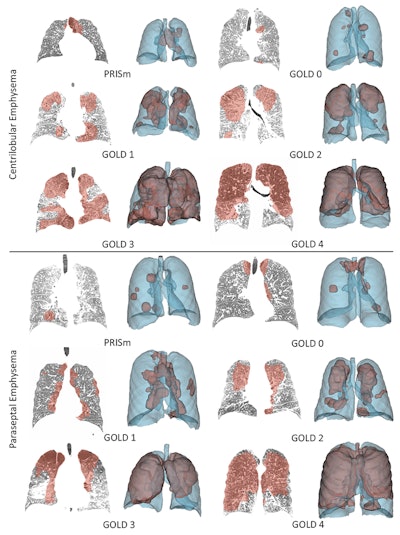
That study used self-supervised deep learning to generate radiomic features that are more generalizable than traditional methods. The approach they developed integrates multimodal data, including CT imaging, genetic and genomic data and personalized biomechanical modeling of the tissue. This enables more accurate disease subtyping and earlier, patient specific projections of COPD progression.
“The material properties of lung tissue change due to disease, but direct measurement of these properties (such as tissue stiffness) is typically not feasible,” Dr. Batmanghelich said.
The aim of the research is to estimate those properties from lung motion, which the researchers hope will yield more interpretable results.
“If we can further link these estimated properties to other patient data, including genetic and genomic measurements, we can develop novel and clinically meaningful multimodal biomarkers for [COPD],” Dr. Batmanghelich said.
While focused on COPD, he said the tools and methods developed through this research could be extended to other pulmonary diseases that affect lung biomechanics.