A phase 2 trial for a potential new treatment for COPD has gotten underway. Upstream Bio, Inc., a company developing treatments for inflammatory diseases, dosed the first patient in its new phase in early July, according to a press release.
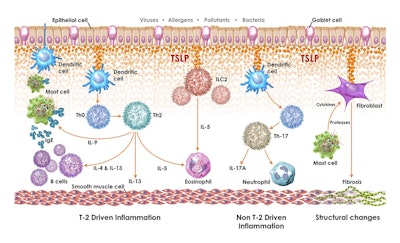
The VENTURE trial is a randomized, double-blind, placebo-controlled trial designed to assess the efficacy and safety of verekitug. Verekitug is a novel antibody antagonist of the thymic stromal lymphopoietin (TSLP) receptor, which is a key driver of inflammatory response in diseases like COPD and asthma.
Aaron Deykin, MD, chief medical officer and head of research and development at Upstream Bio, said there are emerging data indicating that TSLP plays an important role in driving the pathobiology of COPD and the exacerbations that go along with it.
“Given the results observed in our pre-clinical and early clinical studies in asthma, we believe that as the only known biologic in development targeting the TSLP receptor, verekitug may have the potential to advance COPD treatment with less frequent dosing and differentiated efficacy as compared to currently approved biologic therapies for this condition,” he said.
The VENTURE trial includes approximately 670 adults with moderate to severe COPD who will be placed into randomized groups to receive verekitug doses at 100 mg once every 12 weeks, 400 mg once every 24 weeks or placebo over periods between 60 and 108 weeks.
The primary endpoint of the study is the annualized rate of moderate or severe COPD exacerbations. Secondary endpoints include changes in participants’ day-to-day symptoms as well as measures of lung function.
The company said the COPD trial is part of a broader development program for verekitug that includes separate, ongoing phase 2 studies in chronic rhinosinusitis with nasal polyps and severe asthma. Data for those trials are expected in the third quarter of 2025 and the first quarter of 2026, respectively.