Researchers have developed an original model in rats that closely replicates the pathological features and physiological changes associated with human COPD-associated cor pulmonale — changes in the function of the right ventricle of the heart caused by respiratory illnesses. Their study, “Development and Characterization of a Novel Rat Model for Emulating Chronic Obstructive Pulmonary Disease-Associated Cor Pulmonale,” was published in The American Journal of Pathology.
In a news release, researchers said the study details the potential for the model to unravel the complex interactions between lung and heart pathology and improve patient outcomes.
According to the study, approximately 6% of COPD patients develop cor pulmonale each year, which not only increases mortality among patients but also imposes a significant economic burden on society.
Lead investigator Tao Wang, MD, PhD, said this study could help fill a gap in COPD treatment. Dr. Wang is a researcher at State Key Laboratory of Respiratory Services, part of the National Clinical Research Center for Respiratory Diseases at the Guangzhou Institute of Respiratory Health in Guangzhou, China.
“The prognosis for individuals with COPD complicated by cor pulmonale is generally poor, and the existing treatment options are inadequate,” Dr. Wang said. “To address this urgent need for a more accurate animal model of COPD-associated cor pulmonale, we were dedicated to developing a novel rat model to better emulate the human disease, providing valuable tools for future research and therapeutic development.”
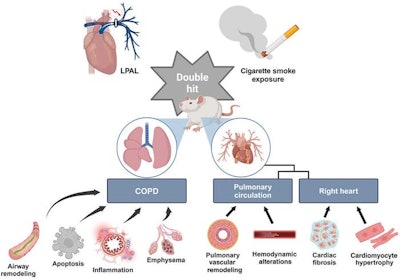
The researchers induced a COPD-associated cor pulmonale disease state in rats by combining chronic cigarette smoke exposure with left pulmonary artery ligation, followed by rigorous physiological, histological and molecular analyses.
Results showed that this model recapitulated the pulmonary dysfunction, emphysema and inflammatory infiltration characteristic of COPD. In addition, it also reproduced key features of cor pulmonale, including right ventricular hypertrophy, fibrosis, capillary rarefaction and hemodynamic changes associated with pulmonary hypertension. The investigators also identified that pathways involving inflammation and oxidative stress may play significant roles in disease progression, highlighting their potential as therapeutic targets.
The model is expected to facilitate the discovery of new molecular insights and the identification of innovative therapeutic targets.