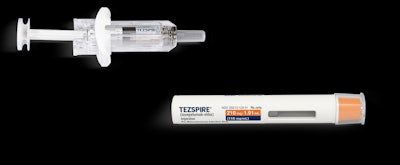
The U.S. Food and Drug Adminstration (FDA) has granted Tezspire (tezepelumab) breakthrough therapy designation as an add-on maintenance treatment for patients with moderate to very severe COPD characterized by an eosinophilic phenotype.
According to the FDA, a breakthrough therapy designation is for drugs that treat serious or life-threatening conditions where primary clinical evidence indicates the drug may demonstrate substantial improvement on a clinically significant endpoint compared to available therapies.
Amgen, the maker of the Tezspire treatment for COPD and asthma, made the announcement during its second-quarter financial report, adding that the decision came after positive data from its COURSE phase 2a trial results, which were announced earlier this year.
The COURSE phase 2a was a proof-of-concept trial that investigated tezepelumab in patients with moderate to very severe COPD across a broad range of eosinophil levels — irrespective of inflammatory drivers, emphysema, chronic bronchitis and smoking status.
This study did not exclude any patients based on their baseline eosinophil count (BEC) and intentionally enrolled patients with a broad range of BECs. Overall, tezepelumab numerically reduced the annualized rate of moderate or severe COPD exacerbations versus placebo by 17%.
The company said it was moving forward with a Phase 3 trial of the drug in April. Tezspire is currently approved by the FDA as an add-on maintenance treatment for adults and children 12 years and older with severe asthma.