The European Patent Office has granted a new patent that covers the use of brilaroxazine for the treatment of pulmonary hypertension (PH) and pulmonary arterial hypertension (PAH) in patients with COPD or sickle cell disease. The treatment, which is manufactured by Reviva Pharmaceuticals, already has similar patents in markets around the world including the U.S., China and Japan.
“The latest patent further secures the broad therapeutic potential of brilaroxazine for inflammatory conditions driven by underlying disruption in serotonin signaling like pulmonary hypertension,” said Laxminarayan Bhat, PhD, founder, president and CEO of Reviva. “Brilaroxazine has demonstrated a favorable clinical safety and tolerability profile in over 800 subjects to date from multiple clinical trials.”
Dr. Bhat said results from the phase 3 RECOVER-1 clinical trial — a large, global study of patients who have schizophrenia — demonstrated that brilaroxazine significantly reduced key proinflammatory biomarkers compared to placebo. Additional research has been completed to assess the efficacy and safety of the drug.
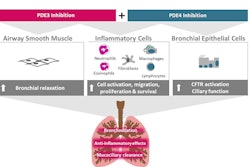
“Building on this promising clinical data, potent anti-inflammatory and antifibrotic activities and significant reduction in pulmonary arterial pressure has been shown in translational animal models for PAH following brilaroxazine treatment,” he said. “We look forward to further evaluating brilaroxazine’s unique, multi-modal mechanism of action with clinical development expansion opportunities in PH and PAH.”