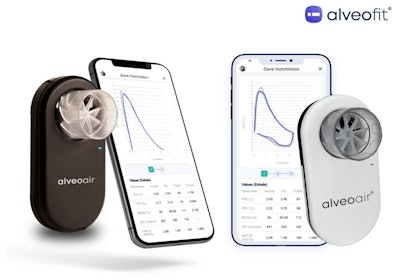
Physicians and patients in the U.S. have a new tool in their arsenal for diagnosing and monitoring lung disorders such as asthma and COPD.
Alveofit Roundworks Technologies’ alveoair spirometer has received clearance from the U.S. Food and Drug Administration for distribution in the U.S. The device is India’s first FDA-cleared, portable digital spirometer and is part of alveofit’s platform that offers real-time, monitoring insights via a mobile phone app to health care professionals and their patients to enable timely interventions in respiratory care.
The company has formed a strategic partnership with AstraZeneca to make the device available to physicians and their patients and has opened an office In New York City.
Dr. Anil Kukreja, vice president of medical affairs and regulatory at AstraZeneca, said the FDA clearance was a “milestone” that will help with the treatment of lung disorders in the U.S. as well as positions alveoair for broader international expansion.
Since achieving certification in India in 2022, alveoair has been adopted by more than 400 health care facilities across India. The company has also partnered with organizations like India Sweden Innovation Centre and NASSCOM Center of Excellence—a technology innovation ecosystem in India which is comprised of startups, enterprises and the government.
“It’s good to see the product mature over the last couple of years and gain acceptance among the providers,” said Sanjeev Malhotra, CEO of NASSCOM COE.