Teva Pharmaceuticals USA, Inc., announced new data on its Digihaler System, which was presented at the American Academy of Allergy, Asthma and Immunology 2023 Annual Meeting in February. The data included findings from the CONNECT2 clinical trial program as well as information on patients and health care professionals' real-world use of the smart inhaler system. The Digihaler System is a smart inhaler system with built-in sensors that provide objective inhaler event data to help patients and their doctors support asthma management and develop personalized treatment plans.
The data was featured in five posters, highlighting:
- Real-world Digihaler System usage patterns for patients
- The relationship between medication adherence and patients’ voluntary responses to a daily self-assessment (DSA) feature
- Barriers, facilitators and HCP recommendations related to implementation of digital inhaler technology
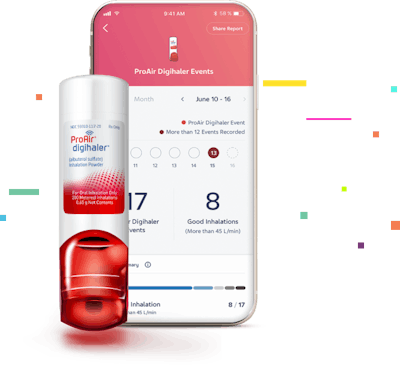
Digihaler is a digital health system comprised of an electronic multidose dry powder smart inhaler, connected app, Digital Health Platform cloud solution and dashboard. Digihaler inhalers have built-in flow sensors that detect, record and store objective data showing how often and how well patients use their inhalers, as measured by inspiratory flow. Patients are able to view their data on the Digihaler app, including inhaler use patterns over time, and can share it with a health care provider to facilitate dialog and personalize treatment discussions. Health care providers can also monitor the data on a dashboard with patient permission.
As per the U.S. Food and Drug Administration (FDA) approved label, there is no evidence that the use of the app leads to improved clinical outcomes, including safety and efficacy.
CONNECT2
The CONNECT clinical program evaluated the potential impact of Digihaler System on patient-provider interactions, adherence over time, inhaler technique and frequency of short-acting beta agonist (SABA) use. The CONNECT2 trial was conducted to assess the role of both the ProAir Digihaler (albuterol sulfate) inhalation powder and AirDuo Digihaler (fluticasone propionate and salmeterol) inhalation powder in the treatment of asthma as part of the Digihaler System.
CONNECT2 was a 24-week open-label, multicenter, randomized, parallel group study that evaluated the impact of this Digihaler System on asthma management compared to standard of care (SoC) in 427 patients with asthma. Participants in the SoC group continued treatment with their current SoC asthma maintenance and reliever medications.
Digihaler integrated inhalers transmit data wirelessly to a smartphone app, which allows for inhaler event tracking. The app includes a voluntary, non-respiratory-specific DSA. The DSA includes questions asking how a patient is feeling on a three-point scale ranging from sad to neutral to happy.
The two CONNECT2 analyses that were presented at AAAAI explored:
- The relationship between SABA use and patient self-assessment responses
- The relationship between medication adherence and patient self-assessment response